Abstract
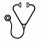
Background
The role of single vs double autologous stem cell transplantation (ASCT) for newly diagnosed (ND) multiple myeloma (MM) continues to be debated in the novel agent era.
Methods
The phase III EMN02/HO95 study was designed to administer 3-4 cycles of bortezomib-cyclophosphamide-dexamethasone as induction therapy for NDMM and afterwards to randomize eligible patients to receive (randomization 1, R1) standard-dose intensification treatment with bortezomib-melphalan-prednisone (VMP) for four 42-day cycles or high-dose intensification treatment with melphalan at 200 mg/m2 (HDM) plus ASCT. A second randomization to receive or not receive consolidation therapy was planned after the intensification phase, followed by lenalidomide maintenance in both arms. In centers committed to a double ASCT policy, patients were randomized (1:1:1) to receive VMP or single ASCT (ASCT-1) or two sequential courses of HDM (administered 2 to 3 months apart) plus double ASCT (ASCT-2) in order to prospectively compare ASCT-1 vs ASCT-2, which was an additional study objective. For this purpose, and for consistency with the primary study end point, progression-free survival (PFS) from R1 was evaluated.
Results
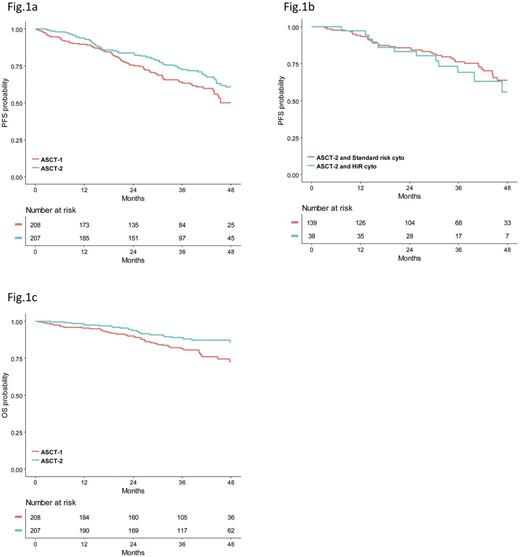
A total of 1503 patients aged ≤65 years were registered and 1192 were eligible for R1. By study design, 618 patients who received the diagnosis of MM in centers with a double ASCT policy were randomly assigned to VMP (n=203) or ASCT-1 (n=208) or ASCT-2 (n=207). 415 of these patients who were randomized to receive ASCT-1 or ASCT-2 were included in the current pre-specified analysis. Median age was 58 years for patients in the ASCT-1 group and 57 years for those in the ASCT-2 group. The frequency of ISS stage III was 19% in both groups. According to IMWG criteria, a high-risk (HiR) cytogenetic profile defined by t(4;14) ± t(14;16) ± del(17p) positivity (HiR-cyto-3) was detected in 26% and 21% of patients who were evaluable in ASCT-1 (80%) and ASCT-2 (86%) groups. The presence of amp(1q) ± del(1p) ± 1 or more of t(4;14), t(14;16) and del(17p) identified a HiR cytogenetic profile (HiR-cyto-5) which was detected in 55% of patients in ASCT-1 arm and 50% of those in ASCT-2 arm. Median follow-up from R1 was 38 (IQR: 29-47) months for the overall patient population (36 and 39 months for patients randomized to ASCT-1 and ASCT-2, respectively). On an intention-to-treat basis, 3-year estimate of PFS was 73% (95% CI=66-79) for ASCT-2 group vs 64% (CI=57-71) for ASCT-1 group (HR=0.70; CI=0.50-0.98; P=0.040), which represented a 30% reduced risk of progression or death in the ASCT-2 group compared with the ASCT-1 group (Fig. 1a). PFS benefit associated with ASCT-2 was confirmed in subgroups of patients with HiR-cyto-3 (HR=0.42; CI=0.21-0.84; P=0.014), revised ISS (R-ISS) stage II+III (HR=0.64; CI=0.43-0.97; P=0.034), age >55 years (HR=0.64; CI=0.43-0.96; P=0.033); HiR-cyto-5 (HR=0.65; CI=0.42-1.01; P=0.059) and best ≥VGPR (HR=0.64; CI=0.44-0.94; P=0.023). Importantly, ASCT-2 overcame the adverse prognosis conferred by HiR-cyto-3 (3-year PFS: 76% vs 69% for patients with standard-risk cytogenetic profile; P=0.482) (Fig. 1b). In particular, 3-year PFS estimate for patients randomized to ASCT-2 and carrying or lacking del(17p) was 72% vs 73%, respectively (P=0.534). The corresponding PFS values in the ASCT-1 group were 43% vs 67%, respectively (P=0.014). In a multivariate Cox regression analysis, randomization to ASCT-2 (HR=0.65; CI=0.44-0.95; P=0.029), R-ISS I (HR=0.60; CI=037-0.98; P=0.042), absence of HiR-cyto-5 (HR=0.35; CI=0.22-0.55; P<0.001) and best ≥VGPR (HR=0.27; CI=0.17-0.44; P<0.001) were the leading independent predictors of PFS. Overall survival (OS) from R1 was significantly prolonged with ASCT-2 as compared with ASCT-1 (3-year rate: 89% vs 82%; HR=0.52; CI=0.31-0.86; P=0.011) (Fig. 1c), a benefit also seen in subgroups of patients with adverse prognosis, including those with R-ISS stage II+III (HR=0.48; CI=0.27-0.86; P=0.013) and HiR-cyto-5 (HR=0.52; CI=0.28-0.98; P=0.042).
Conclusions
Randomization to ASCT-2 was superior over ASCT-1 in terms of prolonged PFS and OS for the overall patient population and for poor prognosis subgroups of patients with advanced R-ISS disease stage and HiR cytogenetic profile. Incorporation of bortezomib into ASCT-2 abrogated the increased risk of progression or death imparted by t(4;14) ± t(14;16) ± del(17p), and in particular by del(17p) positivity.
Gay: Amgen: Honoraria; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria. Bringhen: Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Karyipharm: Membership on an entity's Board of Directors or advisory committees. Musto: janssen: Honoraria; Celgene: Honoraria; Novartis: Honoraria; BMS: Honoraria. Palumbo: Takeda: Consultancy, Employment, Equity Ownership, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding; Binding Site: Research Funding; Sanofi: Consultancy, Honoraria, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Genmab A/S: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Sonneveld: Celgene Corporation, Amgen, Janssen, Karyopharm, PharmaMar, SkylineDx: Honoraria; Celgene, Amgen, Janssen, Karyopharm, Takeda: Consultancy, Honoraria, Research Funding; Celgene Corporation, Amgen, Janssen, Karyopharm, SkylineDx, PharmaMar: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract